Sterile rooms or clean rooms? Contrary to popular belief, the two terms are not interchangeable. Take a deep dive into the subject with KleanLabs, learning about where sterile manufacturing is used, what classifications are applicable and find out about some best practices applied in sterile manufacturing.
What differentiates sterile rooms from clean rooms?
Clean rooms are highly controlled indoor environments where the number of particles and contaminants in the air, the temperature, humidity and pressure are all restricted by predetermined standards, including the number of micron and tenth micron particles. In practice, air entering the clean environment is filtered and controlled to given denominators by the air handling unit equipped with HEPA filters or fan filter units in some cases.
Read about clean room standards and classifications here.
Sterile environments, however, go a step further - they are completely bacteria and microorganism free. Sterile manufacturing is usually required for some very specific tasks such as filling of vaccines.
What industries prefer sterile manufacturing and sterile environments?
Among others, injections, infusions, organ transplant solutions, but also ophthalmic preparations require a sterile environment. For these processes, it is of vital importance that they are not contaminated with microbes during manufacturing, processing or storage. A sterile environment is a clean room where the possibility of this is entirely ruled out, since the system is closed, microorganism cannot be found.

Sterile environments are common in the pharmaceutical and medical field where the presence of microorganism would bear great risk
Classification of sterile manufacturing
We differentiate between two types of sterile manufacturing:
- Terminal sterilization: the product is sterilized before or during packaging, but is not in fact manufactured in a sterile environment. Terminal sterilization can be performed with
- Moist heat: air is removed from the final packaging before the sterilizing steam penetrates.
- Dry heat: sterilized by air filtration during manufacturing, where air can only enter through a HEPA filter. The resulting overpressure in the chamber prevents non-sterile air from entering.
- Aseptic manufacturing: if the product cannot be sterilized by air filtration or in the final phase by moist heat, it must be manufactured in an aseptic environment. The process involves some risk, as some viruses or microplasmas may pass through a filter with a nominal pore size of 0.2 μm (or less). The risk can be mitigated by combining filtration with heat treatment.
Grades of sterile medicinal product manufacturing
| Grade | Maximum number of particles per m3 | Number of viable microorganism per m3 | |
|---|---|---|---|
| 0,5 - 5 μm | |||
| 5 μm | |||
| A | 3500 | 0 | less than 1 |
| B | 3500 | 0 | 5 |
| C | 350 000 | 2000 | 100 |
| D | 3 500 000 | 20 000 | 500 |
Grade D
Grade D environments are used to store equipment when they’re not in use. The staff also starts gowning in this area by covering their hair, beards and putting on general protective clothing.
Grade C
Final sterilization can be performed in environments of grade C classification. Staff can only enter “Zone D” from here, that is, they may not enter directly. In this environment, two-piece protective clothing closed at the wrists and neck is mandatory.
Grade B
This is where aseptic manufacturing takes place, i.e. parts of the manufacturing process that are considered high-risk. Laminar workstations can be classified as “Grade A”, for example, which allow work to be carried out at a higher degree of purity within a given ISO-class cleanroom. It is often sufficient to use a laminar box that provides the right environment for the particular operation.
The presence of personnel, especially during operation, should be minimized. If this is not possible, they can only enter this zone in full protective gear. Visitors and maintainers who are not part of the staff must be strictly supervised.
Best practices of sterile manufacturing
In sterile environments, equipment must also be sterile. This is why every item, from protective clothing to work equipment, requires special attention.
The use of “gray zones” between two clean or sterile rooms can be very beneficial if the classification of the zones is different. The role of these corridors is paramount in minimizing the chances of cross-contamination. Use of pressure cascades is a typical way, meaning that contaminants always flow from the cleanest environment to the least clean zone.
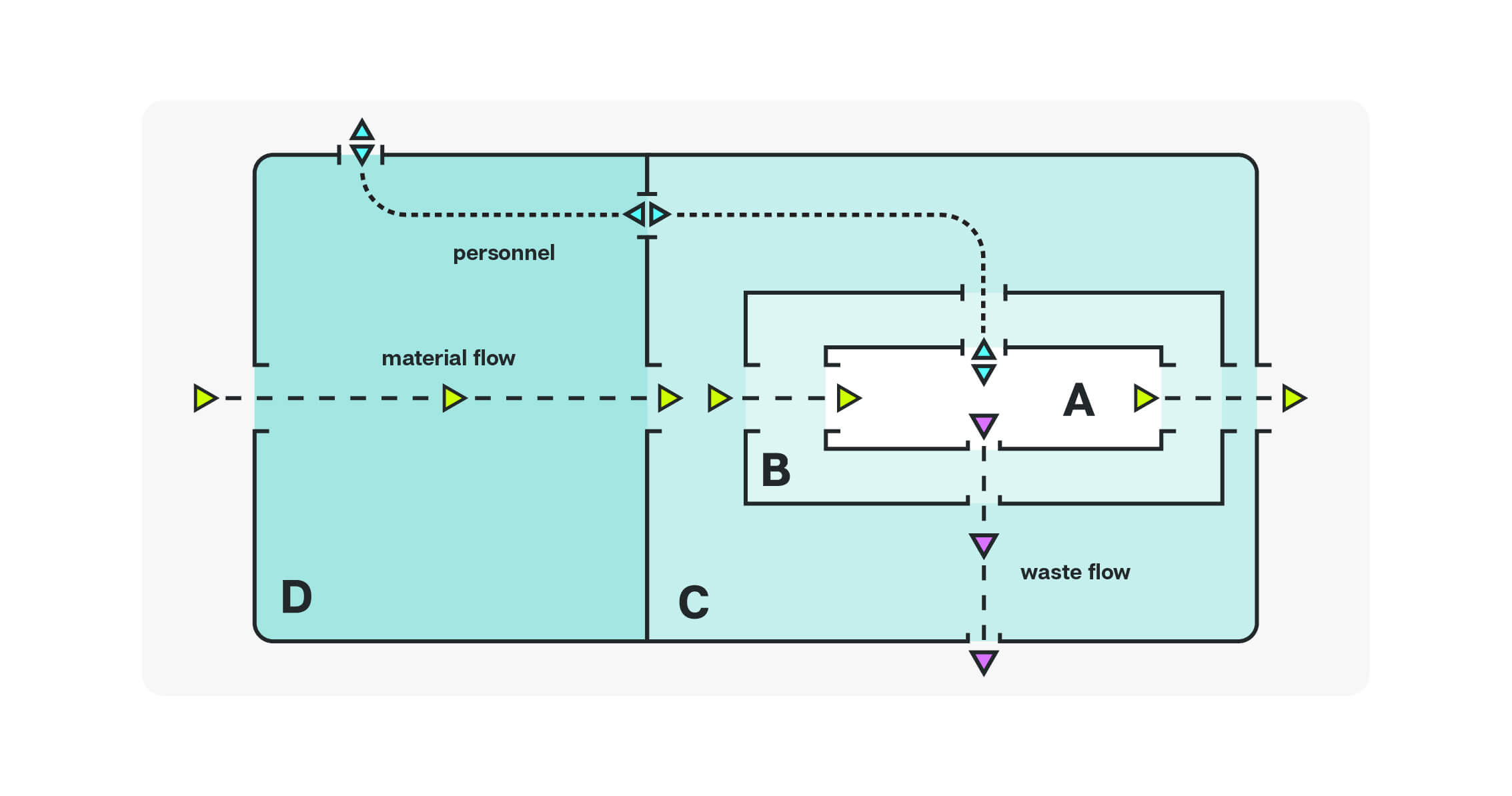
Material flow depicted in a sterile environment
Material flow in these environments must also be designed keeping the sterile grades in mind. Pass boxes can facilitate material and equipment transfer between the cleanest zones without additional staff members having to enter the environment.

Proper gowning is key in both clean and sterile environments
Sterile vs. clean room garments
What makes clean garments clean?
Cleanroom garments must be washed and filtered to 0.002 micron to be considered ready for use. They must be free of excess particles, therefore, must undergo at least three washes. Both inspection and packaging of garments is performed in a clean, controlled environment. They are then sealed in poly bags and double packaged. Following such a meticulous process is key for pharmaceutical and automotive applications.
Of course, not all fabrics are suitable for clean rooms. Some materials shed more than others, emitting excess particulates into the environment. In the case of gloves, for instance, nylon and HPPE are used for this reason.
What makes sterile garments sterile?
On the other hand, sterile garments must also be bacteria free. Microorganisms living with the fabric can be killed by radiation called gamma irradiation processing among others. Such sterilized garments are mainly used in surgical and medical fields.
Practically, any piece of garment can be sterilized without having to be manufactured in a special way. However, in many cases, sterilized garments may still have a high number of particulates on their surface that may not comply with given clean room standards.
Clean and sterile garments meet the requirements of both manufacturing processes. These pieces of clothing are manufactured in a highly controlled clean environment and also undergo sterilization, assuring that they won’t introduce foreign particulates into the controlled environment where they will be used.
Key takeaways
All in all, we can conclude that sterility adds a whole new dimension to clean environments. In case of invasive procedures, certain strains must be protected from other strains. This is why aseptic techniques are essential in some industries such as the medical field or pharmaceutics. However, the required level of sterility may also differ, which can influence the build up of a clean room. While in certain cases a laminar flow must assure the highest level of sterility, the environment where final sterilization may take place has less strict requirements.






